Advanced Spinal Technologies, LLC.
Cunningham BW, Seiber B, Riggleman JR, Van Horn MR, Bhat A: An Investigational Study of a Dual‐layer, Chorion‐free Amnion Patch as a Protective Barrier Following Lumbar Laminectomy in a Sheep Model. J Tissue Eng. Regen Med. 2019;13(9):1664-71.
Wessell A, Flemming C, Caffes N, Johnsen P, Lewis E, Cunningham BW: and Sansur CA: Multi-directional flexibility testing of cortical versus pedicle screw stabilization in the osteoporotic lumbar spine: an in-vitro human cadaveric model. (Submission Date for World Neurosurgery6/2019).
Umekoji H, Cunningham BW, Murgatroyd AA, Sun X, Shirado O, McAfee, PC, Oda H: Spinal kinematics of dynamic posterior stabilization at the superior operative and adjacent levels in lumbar spinal reconstruction: an in vitro human cadaveric model. (Submission Date for The Journal of Neurosurgery – Spine 7/2019).
Cunningham, BW, Trontis AJ, Umekoji H, McAfee PC: A comprehensive biomechanical investigation of anterior lumbar interbody arthrodesis with intracorporeal screw fixation: an in vitro human dadaveric model. (Submission Date for The Journal of Neurosurgery – Spine 7/2019).
Trontis AJ, Cunningham, BW, Mullinix K, Sandhu F: Multidirectional flexibility and fixation properties of occipital bolt versus plate stabilization systems: emphasis on integrity of the osseous structures at the occipital implantation sites – (Submission Date for The Journal of Neurosurgery – Spine 7/2019)
Cunningham BW, Weiner DA, Kretzer RM, Tortolani PJ: Biomechanical properties of direct lateral interbody fusion with supplemental posterior instrumentation for adjacent level reconstruction: an in vitro human cadaveric model. (Submission Date for The Journal of Neurosurgery – Spine 8/2019).
Weiner DA, Cunningham BW, Sun X, McAfee, PC: Biomechanical and radiographic evaluation of an articulating interbody fusion device for TLIF; an in vitro human cadaveric model. (Submission Date for The Journal of Neurosurgery – Spine 10/2019).
The laboratory provides a basic scientific environment in which researchers, clinicians, and private industry corporations share the opportunity to pursue their orthopaedic and neurosurgical based research interests.
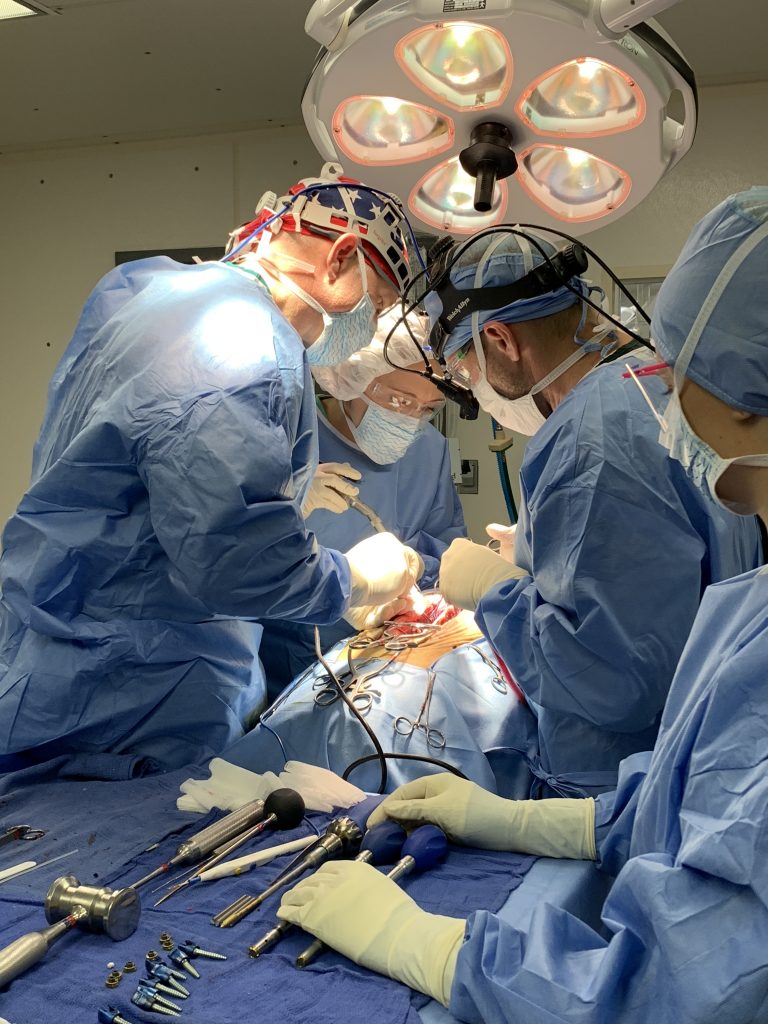

The laboratory facilities are divided into three primary areas: Biomechanics, Histology and Radiography.
Using systematic and scientific methodology, the primary focus of the laboratory is to investigate the biomechanical and biological response to spinal instrumentation, and determine the optimal materials and techniques to achieve stabilization and motion preservation in the spine while providing the utmost quality of life.
Basic Scientific Research
The laboratory collaborates with neurosurgeons and spine surgeons from The Johns Hopkins Hospital, University of Maryland, Union Memorial Hospital, and other prestigious national and international institutions to address basic scientific research issues related to reconstructive spinal surgery.
FDA Pre-Clinical Studies
The laboratory is a Good Laboratory Practices (GLP) compliant facility that performs extensive FDA pre-clinical studies using in-vitro cadaveric and in-vivo animal modeling to investigate new biomechanical and biological concepts in spinal reconstructive surgery. The majority of these studies are performed for private industry on a proprietary basis to support FDA 510K and IDE applications.
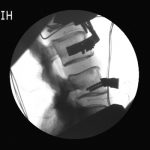
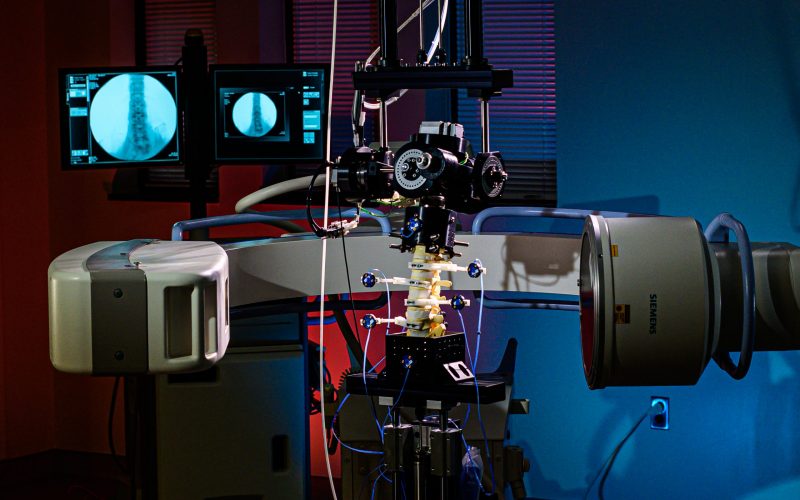 Fluoroscopic video allows for real-time radiographic imaging during range of motion testing.
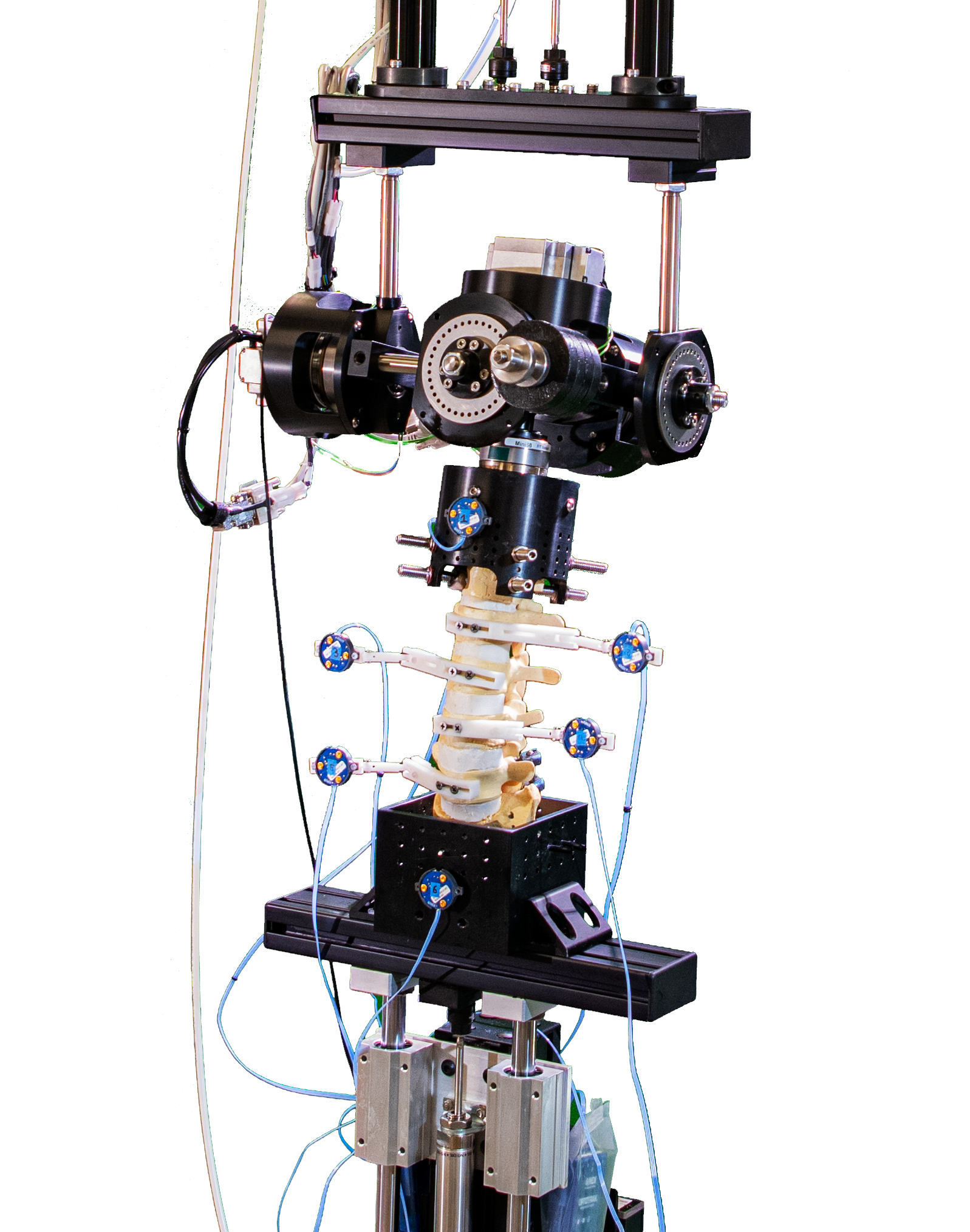
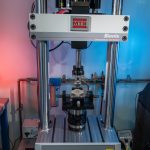
Fluoroscopic video allows for real-time radiographic imaging during range of motion testing. The biomechanical testing laboratory can accommodate complex surgical reconstructions, carried out directly on a custom 6 Degrees of Freedom (6DOF) Spine Simulator.
The biomechanical testing laboratory can accommodate complex surgical reconstructions, carried out directly on a custom 6 Degrees of Freedom (6DOF) Spine Simulator.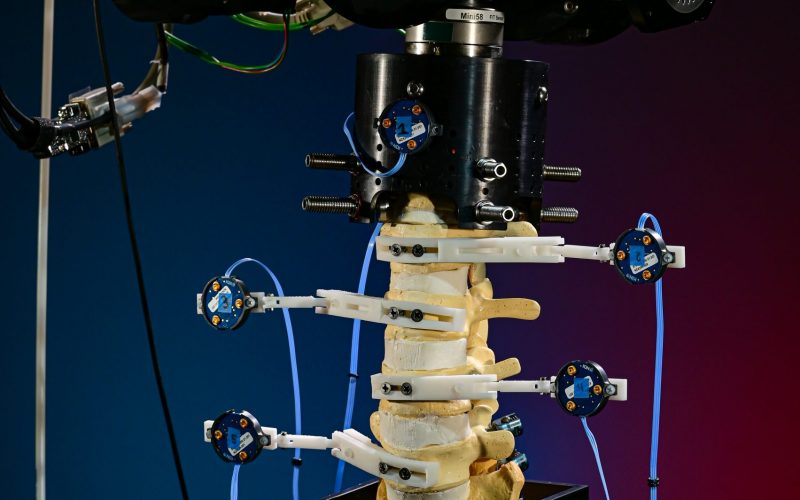 The 6 Degrees of Freedom (6DOF) spine simulator is paired with an optoelectric motion capture system to obtain highly accurate segmental motion tracking during testing.
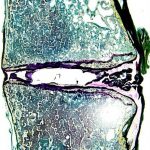
The 6 Degrees of Freedom (6DOF) spine simulator is paired with an optoelectric motion capture system to obtain highly accurate segmental motion tracking during testing. The histology laboratory offers a wide variety of histological preparations for undecalcified bone specimens.
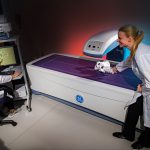
The histology laboratory offers a wide variety of histological preparations for undecalcified bone specimens. DEXA scanning allows the lab to assess bone quality both before and after biomechanical testing.
DEXA scanning allows the lab to assess bone quality both before and after biomechanical testing. Imaging data is viewed, analyzed, and stored in accordance with the highest standards of redundancy and traceability.
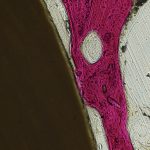
Imaging data is viewed, analyzed, and stored in accordance with the highest standards of redundancy and traceability. The best microscopes Nikon has to offer provide the laboratory with the detailed microscopic views needed for cell counts and histomorphometry calculations.
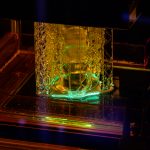
The best microscopes Nikon has to offer provide the laboratory with the detailed microscopic views needed for cell counts and histomorphometry calculations. Formlab’s SLA 3D printer has made 3D printing a reality for the laboratory. Rapid prototyping, preoperative model generation, and in-house fabrication, are a few of the benefits of having in-house 3D printing capabilities.
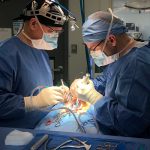
Formlab’s SLA 3D printer has made 3D printing a reality for the laboratory. Rapid prototyping, preoperative model generation, and in-house fabrication, are a few of the benefits of having in-house 3D printing capabilities.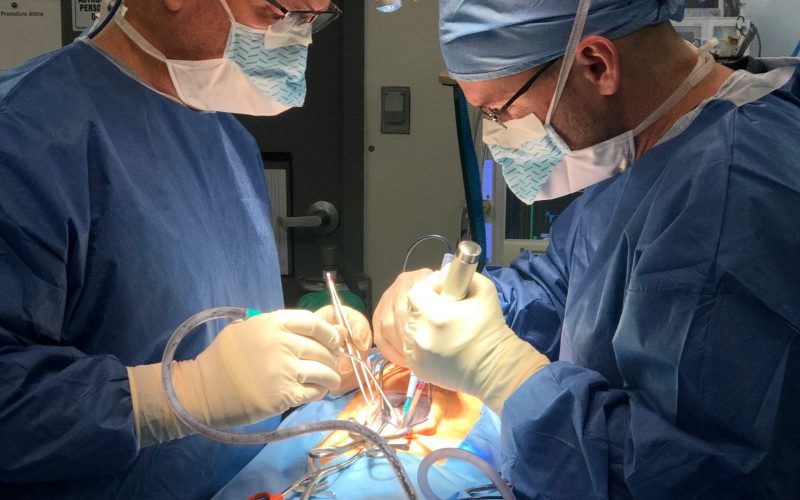 In-vivo biological modeling is made possible through seasoned partnerships with leading surgical facilities.
In-vivo biological modeling is made possible through seasoned partnerships with leading surgical facilities.